Blastocysts Explained: What exactly is a Blastocyst?
Have you ever wondered what exactly a blastocyst is? Well, you’re in the right place. In this article, we will dive into the definition, stages, and implantation of blastocysts, exploring their significance in the early development of mammals.
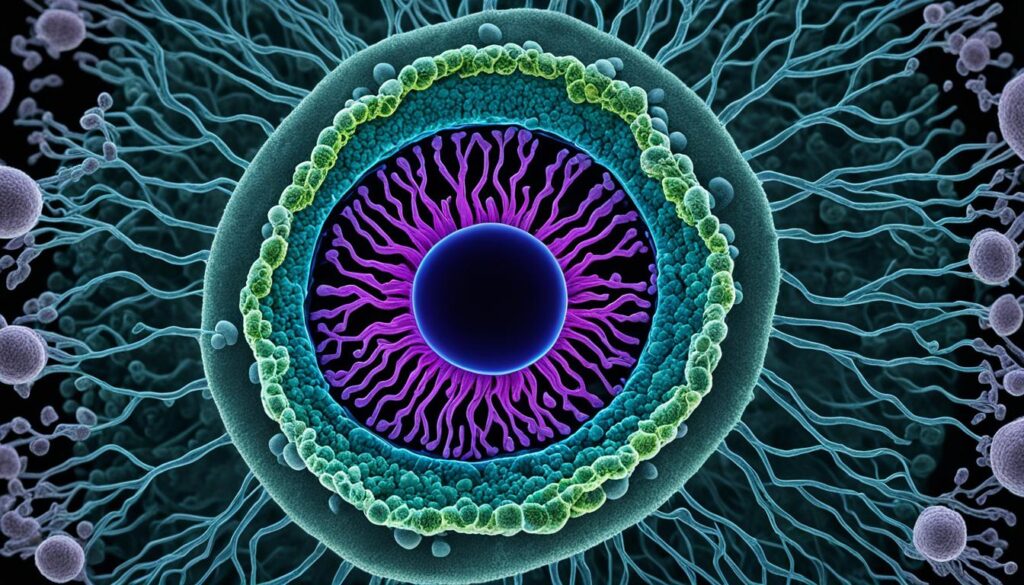
Let’s start with the basics. A blastocyst is a structure that forms during the early stages of embryonic development in mammals. It consists of two main components: the inner cell mass (ICM), also known as the embryoblast, which will later become the embryo itself, and the outer layer of trophoblast cells called the trophectoderm. These trophoblast cells play a crucial role in implantation and the formation of the placenta.
During the formation of a blastocyst, a fluid-filled cavity called the blastocoel also develops. This cavity provides support and nourishment for the growing embryo. In humans, blastocyst formation begins approximately five days after fertilization, and at this stage, it usually consists of around 200-300 cells. With a diameter of about 0.1–0.2 mm, it may seem tiny, but its significance is immense.
Implantation is the next step in the blastocyst’s journey. It involves the attachment of the blastocyst to the uterine wall, where it will continue developing into a fetus. This process requires delicate interactions between the blastocyst and the endometrium of the uterus, facilitated by hormonal changes in the mother’s body. It marks the beginning of gestation and sets the stage for a new life to flourish.
Now that you have a general understanding of blastocysts, let’s delve deeper into their development, structure, and the various applications they have in assisted reproductive technology.
Key Takeaways:
- A blastocyst is a structure formed during the early embryonic development of mammals, consisting of an inner cell mass (ICM) and an outer layer of trophoblast cells.
- The blastocyst also contains a fluid-filled cavity known as the blastocoel.
- Blastocyst formation typically occurs around five days after fertilization, and it plays a crucial role in implantation and further development.
- Implantation is the process in which the blastocyst attaches to the uterine wall, marking the beginning of gestation.
- Blastocysts have various applications in assisted reproductive technology, including in vitro fertilization (IVF) and the study of embryonic stem cells.

Blastocyst Development and Structure
During the early stages of embryonic development, the formation of a blastocyst is a crucial milestone. This process takes place between 5 and 9 days after conception.
After fertilization, the morula, which is a ball of cells, transforms into a blastocyst. The blastocyst has a unique structure consisting of two main components: the inner cell mass (ICM) and the outer trophectoderm cells.
The ICM plays a vital role in blastocyst development. It gives rise to the primitive endoderm, a layer of cells that will form the yolk sac and contribute to the development of organs such as the digestive system. Additionally, the ICM is responsible for the formation of the embryo itself.
On the other hand, the trophectoderm cells surround the ICM and will eventually differentiate into the placenta and other fetal membranes. These structures are essential for the proper nourishment and support of the developing embryo.
Another key feature of the blastocyst is the presence of a fluid-filled cavity called the blastocoel. This cavity provides a protective environment for the developing embryo and enables its further growth and differentiation.
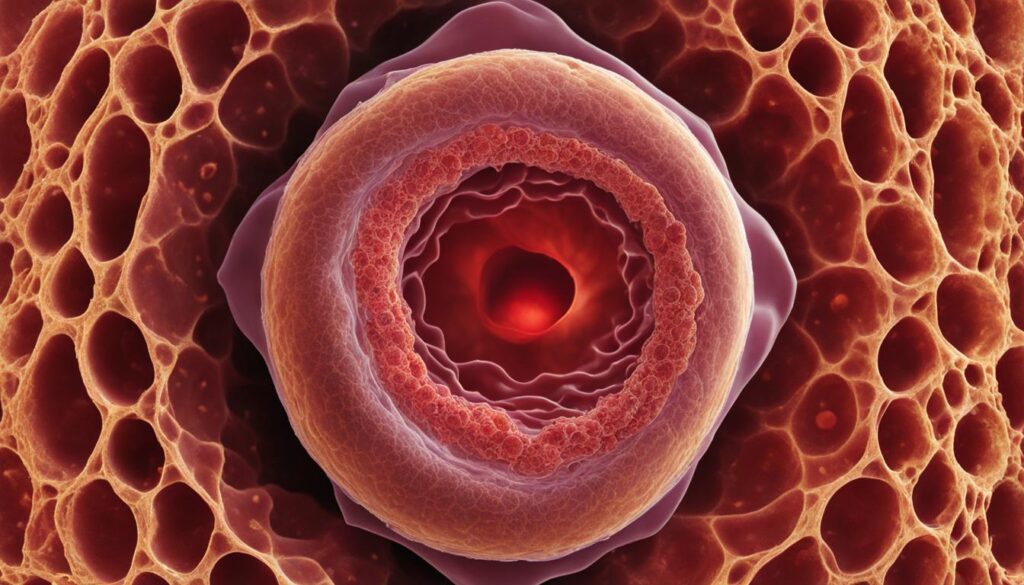
The structure of the blastocyst is critical for its successful implantation into the uterine wall. It ensures that the embryo has the necessary components for further development and interaction with the maternal environment.
Blastocyst Implantation
Blastocyst implantation is a critical step in the early development of the embryo. It involves the attachment of the blastocyst to the endometrium of the uterine wall. Implantation is made possible by changes in both the blastocyst and endometrial wall.
The zona pellucida surrounding the blastocyst breaks down, allowing the embryo to attach to the uterine wall. Hormonal changes in the mother, specifically a peak in luteinizing hormone (LH), prepare the endometrium for implantation.
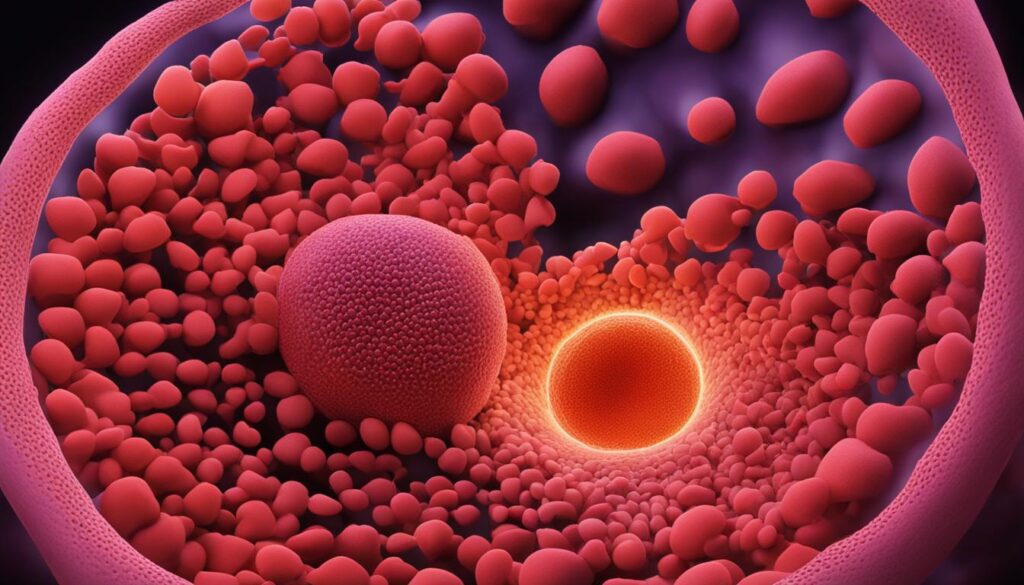
Trophoblast cells in the blastocyst secrete enzymes and other factors to facilitate the embedding of the blastocyst into the uterine wall. This process marks the beginning of gestation.
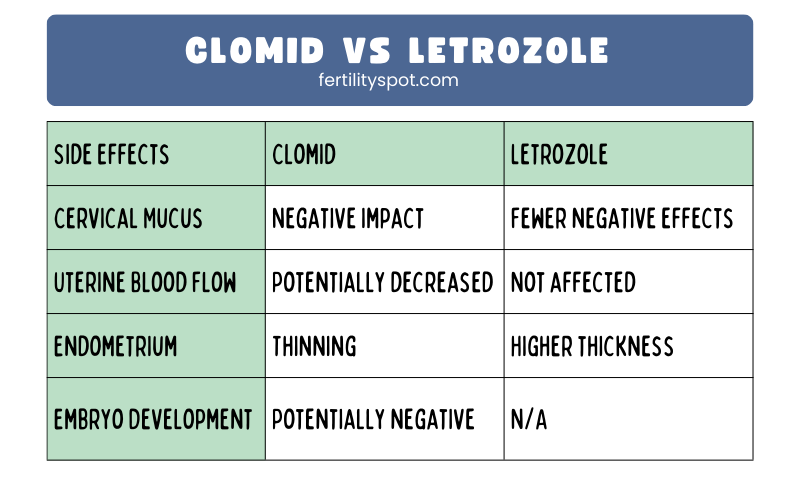
| Blastocyst Implantation | |
|---|---|
| Process | The blastocyst attaches to the endometrium of the uterine wall |
| Changes | Zona pellucida breakdown, hormonal changes in the mother |
| Role | Facilitates the embedding of the blastocyst into the uterine wall |
This crucial step in embryonic development sets the stage for further growth and development of the fetus. Understanding blastocyst implantation sheds light on the intricate processes that occur in early pregnancy.
Blastocyst Uses in Assisted Reproductive Technology
Assisted reproductive technology (ART) has revolutionized the field of fertility treatment, and blastocysts play a crucial role in its success. In vitro fertilization (IVF) is a commonly used ART procedure that involves the cultivation of blastocysts before transferring them into the uterus.
The extended culture of blastocysts for five days allows for a more viable method of embryo selection. This is because blastocysts are more developed and have a higher chance of successful implantation compared to earlier-stage embryos. By choosing blastocysts for transfer, the chances of achieving a pregnancy and a healthy birth are significantly improved.
The inner cell mass (ICM) within the blastocyst is a rich source of embryonic stem cells. These cells have unique properties that make them valuable in various regenerative medicine applications, offering the potential to treat a wide range of diseases and conditions. Many ongoing studies explore the therapeutic potential of embryonic stem cells derived from blastocysts, making them a crucial resource in the field of stem cell therapies.
To facilitate the hatching of the blastocyst from the zona pellucida (an outer protective layer), a technique called assisted zona hatching may be employed during IVF procedures. This process involves creating a small hole or thinning the zona pellucida, enhancing the blastocyst’s ability to implant into the uterine lining.
Blastocyst Uses in Assisted Reproductive Technology:
- Cultivating blastocysts for extended periods allows for better embryo selection and improves the chances of successful implantation.
- The inner cell mass (ICM) of blastocysts is a valuable source of embryonic stem cells that have various applications in regenerative medicine.
- Assisted zona hatching techniques aid the blastocyst in breaking free from the zona pellucida and facilitate implantation during IVF procedures.
By harnessing the potential of blastocysts, assisted reproductive technology has provided countless couples with hope and the opportunity to realize their dreams of starting a family.
| Blastocyst Uses in Assisted Reproductive Technology | Description |
|---|---|
| Extended Culture | Blastocysts are cultured for five days, allowing for better embryo selection and increased chances of successful implantation. |
| Embryonic Stem Cells | The inner cell mass (ICM) of blastocysts is a source of embryonic stem cells, which have various applications in regenerative medicine. |
| Assisted Zona Hatching | Techniques such as assisted zona hatching aid the blastocyst in breaking free from the zona pellucida, enhancing its ability to implant during IVF procedures. |
Blastocyst Grading and Classification
Blastocysts go through a grading and classification process based on their developmental stage and quality. This evaluation helps determine their potential for successful pregnancy and assists in decision-making during assisted reproductive technology procedures. Let’s take a closer look at the different aspects of blastocyst grading and classification.
Blastocyst Expansion Grades
Blastocyst expansion is graded on a scale ranging from 1 to 6, with 6 being the highest grade. This grading system indicates the level of growth and development achieved by the blastocyst. Higher grades signify more advanced expansion, which is a positive indicator of embryo quality and viability.
Inner Cell Mass (ICM) and Trophectoderm Grading
The inner cell mass (ICM) and trophectoderm are additional components that are graded for quality. The ICM grading determines the potential of the mass to form the fetus, with grade A being the highest quality and grade B being acceptable. The trophectoderm grading assesses the outer layer’s ability to develop into the placenta and other vital fetal membranes, with grade A indicating the best quality.
The combined ICM and trophectoderm grading helps embryologists select high-quality blastocysts for transfer. Embryos with higher grades have a greater chance of successful implantation and ultimately leading to a healthy pregnancy.
Subjectivity in Blastocyst Grading
It’s important to note that blastocyst grading is a subjective process and can vary among different in vitro fertilization (IVF) programs. While some criteria may be universally accepted, slight variations in assessment methods may exist. Therefore, it’s essential to work closely with fertility experts and embryologists who are experienced in blastocyst grading to ensure accurate evaluations and recommendations for embryo transfer.
Remember that blastocyst grading is just one factor considered in the overall assessment of embryo quality. Other factors, such as maternal age, previous IVF outcomes, and genetic testing results, are also taken into account to make informed decisions regarding embryo selection and transfer.
Blastocyst Grading Criteria
| Grade | Expansion Level | Inner Cell Mass (ICM) | Trophectoderm |
|---|---|---|---|
| A | Expanding, full cavity | Cohesive, tightly packed | Many cells forming a cohesive layer |
| B | Expanding, partial cavity | Loosely grouped cells | Fewer cells forming a less cohesive layer |
| C | Early blastocyst, no cavity | Loosely grouped cells | Few cells forming a less cohesive layer |
| D | Morula, no blastocoel | Cells compacted, no defined ICM | Cells forming a less cohesive layer |
| Early Blastocyst | Beginning of cavity formation | Cells beginning to differentiate | Cells starting to form trophectoderm |
| Morula | Multicellular, no cavity | Cells undifferentiated | Cells forming a solid mass |
Understanding blastocyst grading and classification helps fertility specialists and patients make informed decisions regarding embryo transfer and the chances of successful implantation. It is an important aspect of assisted reproductive technology that contributes to increasing the success rates of IVF procedures.
Blastocyst Collection and Manipulation
Collecting blastocysts is an essential step in reproductive biotechnology and research. In laboratory settings, blastocysts can be obtained from the reproductive tract of superovulated donors. This process allows for the collection of multiple blastocysts for various applications.
Once collected, blastocysts can be manipulated and studied using advanced techniques. One such technique is immunosurgery, which involves the selective removal of specific cells from the blastocyst. This method allows researchers to isolate and study individual cell populations, such as the inner cell mass (ICM) or trophectoderm.
Microsurgery is another technique used for blastocyst manipulation. With microsurgery, precise and delicate procedures can be performed on the blastocyst, enabling the isolation or modification of specific cell types. These manipulations provide valuable insights into the developmental processes of the blastocyst.
“Blastocyst manipulation techniques like immunosurgery and microsurgery are essential tools in reproductive biology research. They allow us to study the intricate processes occurring within the blastocyst and gain a deeper understanding of early embryonic development.” – Dr. Emily Carter, Reproductive Biologist
The ability to collect and manipulate blastocysts opens up new possibilities in various research and clinical applications. Scientists can utilize these techniques to investigate gene expression, cell fate determination, and developmental pathways within the blastocyst. This knowledge contributes to advancements in reproductive technologies, stem cell research, and cloning methods.
Techniques for Blastocyst Collection and Manipulation
| Techniques | Description |
|---|---|
| Superovulation and Collection | Induction of multiple ovulations followed by the collection of blastocysts from donors. |
| Immunosurgery | Selective removal of specific cells or cell populations from the blastocyst using antibody-assisted techniques. |
| Microsurgery | Precision manipulation of the blastocyst at the cellular level using specialized microsurgical tools. |
The collection and manipulation of blastocysts play a crucial role in advancing our understanding of early embryonic development and reproductive technologies. These techniques provide valuable insights into the complex processes occurring within the blastocyst and pave the way for future breakthroughs in reproductive biology.
Blastocysts and Cloning
When it comes to cloning, blastocysts play a significant role in the process. One of the cloning techniques that involve blastocysts is somatic cell nuclear transfer (SCNT). In SCNT, the nuclei from donor cells are transferred into enucleated metaphase II oocytes. These oocytes are then cultured until they reach the blastocyst stage for further development or transfer.
“The use of blastocysts derived from SCNT embryos has been instrumental in generating cloned animals,” says Dr. Jane Thompson, an expert in reproductive biotechnology. “The successful formation and development of blastocysts have paved the way for significant advancements in cloning.”
Cloning experiments often rely on the successful formation and manipulation of blastocysts. These experiments typically involve the transplantation of blastocysts into surrogate mothers for gestation and birth. Scientists have used blastocysts in cloning to produce genetically identical animals, contributing to our understanding of reproductive biotechnology and its potential applications.
Nuclear Transfer
In nuclear transfer, the genetic material from a donor cell is transferred into an egg cell, which has had its nucleus removed. This process results in an embryo with the genetic characteristics of the donor cell. Researchers have used nuclear transfer techniques, such as SCNT, to clone various animal species, including sheep (Dolly the sheep) and monkeys.
Here is an example of nuclear transfer for cloning purposes:
| Step | Description |
|---|---|
| 1 | Retrieve an oocyte and remove its nucleus. |
| 2 | Take a somatic cell (e.g., skin cell) from the donor animal. |
| 3 | Fuse the somatic cell with the enucleated oocyte. |
| 4 | Culture the resulting embryo until it reaches the blastocyst stage. |
| 5 | Transfer the blastocyst into a surrogate mother for gestation. |
| 6 | Monitor the development and birth of the cloned animal. |
The successful formation and development of blastocysts in the context of cloning have opened up new possibilities in reproductive biotechnology and have sparked further research into the field.
Stay tuned for the next section where we’ll explore the grading and classification of blastocysts in detail.
Conclusion
In conclusion, the blastocyst is a crucial stage in the early development of mammals. Its formation, implantation, and grading are key factors in assisted reproductive technology and research. The use of blastocysts in in vitro fertilization (IVF) has greatly improved the chances of successful pregnancies, and the study of embryonic stem cells derived from blastocysts has opened up new possibilities in regenerative medicine.
Understanding the blastocyst and its functions is essential in the broader context of pregnancy and embryology. It allows us to gain insights into the complex processes that occur during early development and enables us to better assist couples struggling with infertility. By manipulating and studying blastocysts, researchers can further advance our knowledge in reproductive biotechnology and improve our techniques for assisted reproduction.
As the field of reproductive biotechnology continues to evolve, the blastocyst remains a focal point of scientific investigation. Its structure, development, and implantation mechanisms provide valuable information that can lead to enhanced fertility treatments and improved outcomes for individuals and couples alike. The blastocyst truly represents the beginning of life and holds immense potential for advancements in reproductive medicine.
FAQ
What is a blastocyst?
A blastocyst is a structure formed in the early embryonic development of mammals. It consists of an inner cell mass (ICM) known as the embryoblast, which forms the embryo, and an outer layer of trophoblast cells called the trophectoderm.
What are the stages of blastocyst development?
Blastocyst development occurs between 5 and 9 days after conception. After fertilization, the morula transforms into a blastocyst, which consists of the inner cell mass (ICM) and the outer trophectoderm cells. The blastocyst also has a fluid-filled cavity called the blastocoel.
How does blastocyst implantation occur?
Blastocyst implantation involves the attachment of the blastocyst to the endometrium of the uterine wall. Changes in both the blastocyst and endometrial wall make this possible. The blastocyst secretes enzymes and factors to facilitate its embedding into the uterine wall.
What are the uses of blastocysts in assisted reproductive technology?
Blastocysts are cultured before transfer into the uterus in in vitro fertilization (IVF). The inner cell mass of blastocysts is a source of embryonic stem cells, which have broad applications in stem cell therapies. Assisted zona hatching can facilitate the hatching of the blastocyst from the zona pellucida.
How are blastocysts graded and classified?
Blastocysts can be graded based on their developmental stage and quality. Expansion grades range from 1 to 6, with higher grades indicating more advanced development. The inner cell mass (ICM) and trophectoderm are graded based on their quality, with grades A and B being desirable.
How are blastocysts collected and manipulated?
Blastocysts can be collected from the reproductive tract of superovulated donors. In the laboratory, blastocysts are formed through culture systems using fertilized eggs. Techniques such as immunosurgery and microsurgery can be used to isolate inner cell mass and trophectoderm cells for study or manipulation.
What is the role of blastocysts in cloning?
Blastocysts play a significant role in cloning. Cloning techniques involve the transfer of nuclei from donor cells into enucleated oocytes. Blastocysts derived from these embryos can be used to generate cloned animals.
Conclusion
The blastocyst is a crucial stage in the early embryonic development of mammals. Understanding blastocyst formation, implantation, grading, and manipulation is essential in the field of assisted reproductive technology and research.



Responses